
π‐Conjugated Donor Polymers: Structure Formation and Morphology in Solution, Bulk and Photovoltaic Blends - Hildner - 2017 - Advanced Energy Materials - Wiley Online Library

Significant Enhancement of the Electrical Conductivity of Conjugated Polymers by Post-Processing Side Chain Removal | Journal of the American Chemical Society

Polymers | Free Full-Text | Recent Development on Narrow Bandgap Conjugated Polymers for Polymer Solar Cells

Low Band Gap Conjugated Semiconducting Polymers - Scharber - 2021 - Advanced Materials Technologies - Wiley Online Library

Precision Synthesis of Various Low‐Bandgap Donor–Acceptor Alternating Conjugated Polymers via Living Suzuki–Miyaura Catalyst‐Transfer Polymerization - Kim - 2022 - Angewandte Chemie International Edition - Wiley Online Library
![Low Band Gap Donor–Acceptor Conjugated Polymers with Indanone-Condensed Thiadiazolo[3,4-g]quinoxaline Acceptors,Macromolecules - X-MOL Low Band Gap Donor–Acceptor Conjugated Polymers with Indanone-Condensed Thiadiazolo[3,4-g]quinoxaline Acceptors,Macromolecules - X-MOL](https://xpic.x-mol.com/20190809%2F10.1021_acs.macromol.9b00834.jpg)
Low Band Gap Donor–Acceptor Conjugated Polymers with Indanone-Condensed Thiadiazolo[3,4-g]quinoxaline Acceptors,Macromolecules - X-MOL
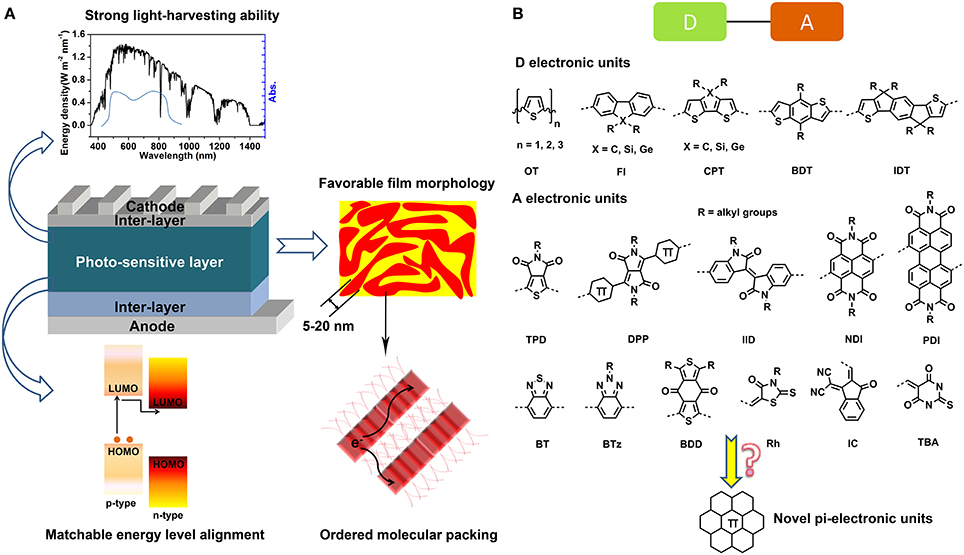
Frontiers | BN Embedded Polycyclic π-Conjugated Systems: Synthesis, Optoelectronic Properties, and Photovoltaic Applications

Very Low Band Gap Thiadiazoloquinoxaline Donor–Acceptor Polymers as Multi-tool Conjugated Polymers | Journal of the American Chemical Society
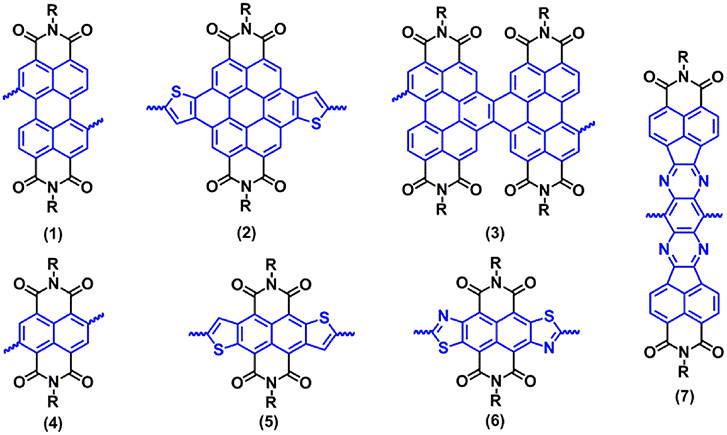
Frontiers | Low Bandgap Donor-Acceptor π-Conjugated Polymers From Diarylcyclopentadienone-Fused Naphthalimides
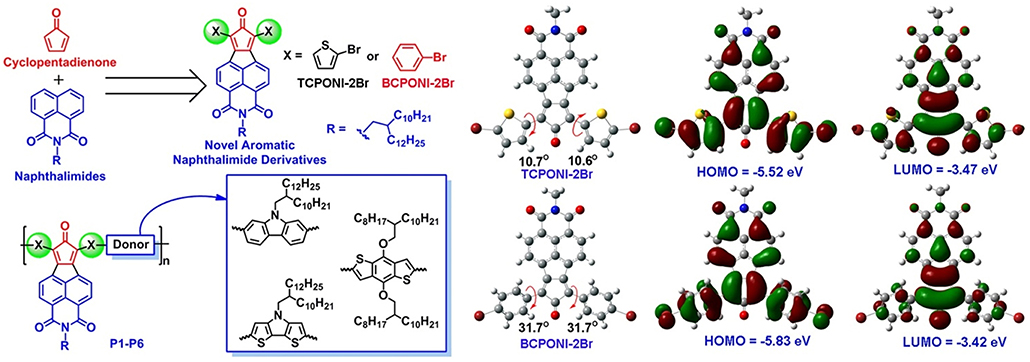
Frontiers | Low Bandgap Donor-Acceptor π-Conjugated Polymers From Diarylcyclopentadienone-Fused Naphthalimides
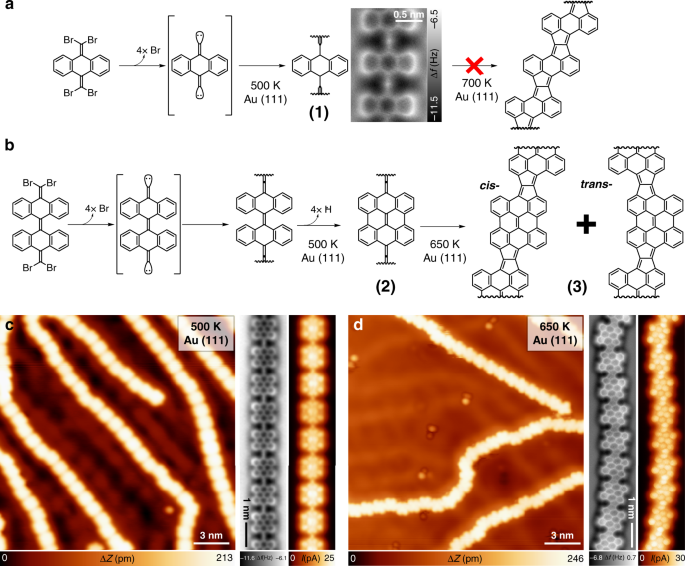
Tailoring π-conjugation and vibrational modes to steer on-surface synthesis of pentalene-bridged ladder polymers | Nature Communications
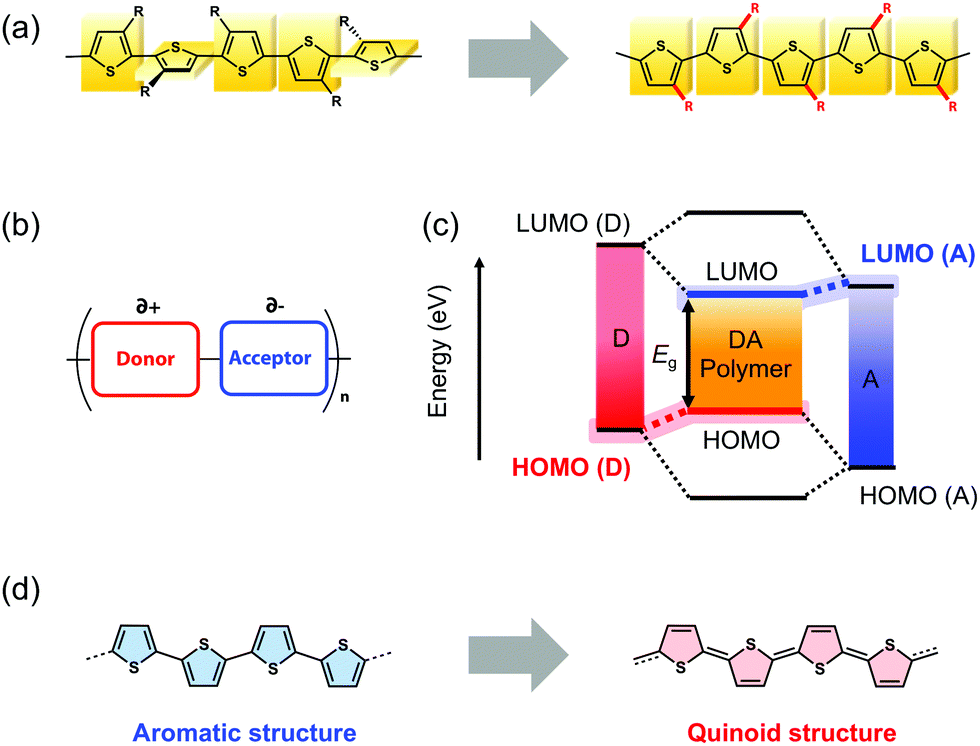
Small-bandgap quinoid-based π-conjugated polymers - Journal of Materials Chemistry C (RSC Publishing) DOI:10.1039/D0TC01041C
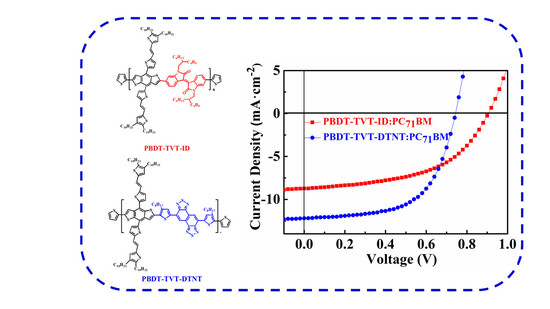
Polymers | Free Full-Text | Synthesis and Photovoltaic Effect of Electron-Withdrawing Units for Low Band Gap Conjugated Polymers Bearing Bi(thienylenevinylene) Side Chains

Low-Band gap Conjugated Polymers with Strong Absorption in the Second Near-Infrared Region Based on Diketopyrrolopyrrole-Containing Quinoidal Units | Macromolecules

Impact of polymorphism on the optoelectronic properties of a low-bandgap semiconducting polymer | Nature Communications

Controlling Molecular Mass of Low-Band-Gap Polymer Acceptors for High-Performance All-Polymer Solar Cells - ScienceDirect

Narrow-band gap Benzodipyrrolidone (BDPD) based donor conjugated polymer: A theoretical investigation - ScienceDirect



